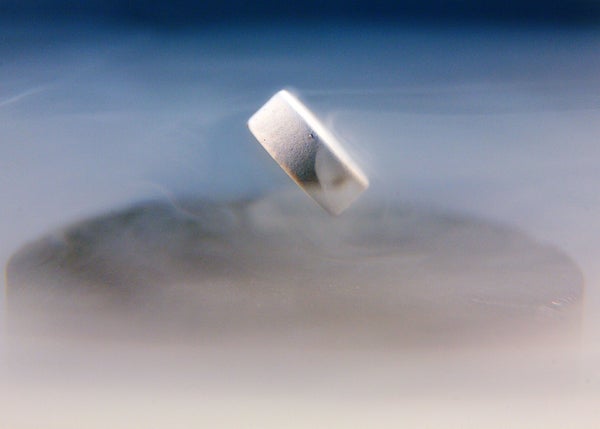
A Nature retraction last week has put to rest the latest claim of room-temperature superconductivity — in which researchers said they had made a material that could conduct electricity without producing waste heat and without refrigeration.
The retraction follows the downfall of an even more brazen claim about a supposed superconductor called LK-99, which went viral on social media earlier this year.
Despite these high-profile setbacks, superconductivity researchers say the field is enjoying somewhat of a renaissance (see ‘Timeline: Superconductivity milestones’). “It’s not a dying field — on the contrary,” says Lilia Boeri, a physicist who specializes in computational predictions at the Sapienza University of Rome. The progress is fuelled in part by the new capabilities of computer simulations to predict the existence and properties of undiscovered materials.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Much of the excitement is focused on ‘super-hydrides’— hydrogen-rich materials that have shown superconductivity at ever-higher temperatures, as long as they are kept at high pressure. The subject of the retracted Nature paper was purported to be such a material, made of hydrogen, lutetium and nitrogen. But work in the past few years has unearthed several families of materials that could have revolutionary properties. “It really does look like we’re on the hairy edge of being able to find a lot of new superconductors,” says Paul Canfield, a physicist at Iowa State University in Ames and Ames National Laboratory.
Surfing electrons
Superconductivity arises when electrons in a solid combine to form ‘Cooper pairs.’ This enables many more electrons than usual to move in sync inside the material, which in turn enables the electrons to carry currents without producing waste heat.
In ‘conventional’ superconductors, electrons form Cooper pairs when nudged together by vibrations in the material — mechanical waves that the Cooper pairs ride like surfers on a wave. Until the mid-2000s, researchers generally thought that this mechanism would work only at extremely low temperatures, up to around 40 kelvin. Superconductors made of a single element all require temperatures lower than 10 kelvin to exhibit this property. Magnesium diboride, a conventional superconductor discovered in 2001 by a team led by Jun Akimitsu at Okayama University in Japan, raised the record for the highest temperature to 39 kelvin.
The basis for super-hydrides was laid out in 2004, when the late theoretical physicist Neil Ashcroft predicted that certain elements would form compounds with hydrogen that could superconduct at much higher temperatures than could any other material, if put under enough pressure to force the hydrogen atoms closer together.
According to Ashcroft’s theory, the proximity of the hydrogen atoms would increase the frequency of mechanical vibrations, which would enable the material to get warmer while retaining its superconductivity. But there was a catch: to even exist, some of these materials would require pressures comparable to those in Earth’s core.
Advances in carrying out high-pressure experiments on tiny samples inside a diamond anvil — and measuring their outcomes — led to a breakthrough in 2015, when physicist Mikhail Eremets at the Max Planck Institute for Chemistry in Mainz, Germany, and his collaborators first demonstrated superconductivity in a super-hydride, hydrogen sulfide. Since then, scientists have predicted the existence of several other superconducting materials in this family — some of which have been found, including calcium-based cage-like structures called clathrates.
At present, the ‘hottest’ superconductor of any kind is considered to be lanthanum decahydride, a member of the super-hydride class that is proven to be a high-pressure, conventional superconductor at temperatures of up to at least 250 kelvin.
Advanced simulations
Eremets and others say that the interplay of theory, simulation, materials synthesis and experiment has been crucial to progress. Beginning in the early 2000s, it became possible for simulations to predict whether a material with a certain crystal structure and chemical composition could be a superconductor, and at what temperatures it could exhibit this property. But the next major shift was the introduction of algorithms later that decade that could predict not just the properties of a material, but what materials can form from a given mix of elements. “Until then, a crucial bit was missing: understanding whether a compound can form in the first place,” says Boeri.
The discovery in 2015 that hydrogen sulfide is a superconductor was consistent with computer simulations conducted the year before. Without rapid advances in structure prediction, the discovery of hydrogen-rich superconductors “probably would have not happened for another century,” says Artem Oganov, a materials scientist at the Skolkovo Institute of Science and Technology in Moscow, who has pioneered structure-prediction algorithms. His ‘evolutionary’ algorithms, in particular, find the configuration of atoms with the lowest energy — and therefore best chance to form and remain stable — at a given pressure.
Simulations are especially crucial for predicting the behaviour of materials at high pressures, under which atoms are pushed so close to one another that they begin to interact not only through their outer electrons, but also with more inner ones, throwing chemistry-textbook dogma out of the window. An example of this is lithium hexahydride, which can exist only at high pressures. “Anybody in general-chemistry class would tell you that something like LiH6 cannot be stable,” says Eva Zurek, a computational chemist at the University at Buffalo in New York.
This article is reproduced with permission and was first published on November 16, 2023.